Continuous Amnioinfusion in Women With Pprom at Periviable Gestational Ages
Abstract
Purpose
Treatment of mid-trimester classic preterm premature rupture of membranes (PPROM) with systemic antibiotics has limited success in the prevention of chorioamnionitis, funisitis and fetal inflammatory response syndrome because of very low transplacental passage.
Methods
Here we report a case of PPROM at 18 weeks gestation with anhydramnion colonized by multi-resistant Escherichia coli (E. coli). A catheter system was implanted at 23/2nd weeks gestation, enabling long-term continuous lavage of the amniotic cavity with Amnion Flush Solution (100 ml/h combined with intraamniotic meropenem application).
Results
The patient gave birth to a preterm male infant at 28/3rd without any signs of infection. In a follow-up examination at 24 months, there was no neurological disturbance or developmental delay.
Conclusion
The classic PPROM with multi-resistant E. coli colonization could be treated with continuous amnioinfusion and meropenem.
Introduction
The 'classic' mid-trimester preterm premature rupture of membranes (PPROM) with oligo/anhydramnion is associated with a very high neonatal mortality rate as well as an increased risk of long- and short- term severe neonatal morbidity [1, 2]. The bacteria rapidly colonize surfaces of amniotic membranes, the umbilical cord and the fetus [2]. Expectant management, broad-spectrum antibiotics and antenatal corticosteroids are routinely used in classic PPROM cases with very limited success [1,2,3,4]. Gomez et al. [5] reported that antibiotics failed to eliminate the amniotic infection in 83% of PPROM cases.
The lavage effect of continuous amnioinfusion could prevent the fetus and amniotic cavity from bacterial colonization, reduce the inflammatory response mediated by cytokines and protect the neonate from major complications, such as pulmonary hypoplasia, sepsis, cerebral palsy and joint deformities [2, 6,7,8,9]. Here we report the management of a classic PPROM and multi-resistant Escherichia coli (E. coli) (3-MRGN) infection with continuous amnioinfusion and intraamniotic administration of meropenem.
Case
An 18-year-old first gravida was referred to our clinic because of classic PPROM since the 18th week of gestation (WG). On admission at 23/0 WG, she complained about massive loss of amniotic fluid. The PPROM was additionally confirmed by positive test of PAMG-1, (AmniSure®, Qiagen Company, France) and anhydramnion. The estimated fetal weight was 336 g. The patient received a single course of betamethasone (12 mg daily for two days; Celestan®, Essex Pharma, Munich, Germany) for prophylaxis of neonatal respiratory distress syndrome. Ampicillin (1000 mg; intravenous) three times daily (Ratiopharm GmbH, Ulm, Germany) and one application of azithromycin (500 mg; per os) (Hexal AG, Holzkirchen, Germany) were used according to the German guidelines [3]. A cervical bacterial swab was performed weekly (Table 1), C-reactive protein (CRP), leucocytes and Interleukin-6 were monitored and controlled daily (Fig. 1).
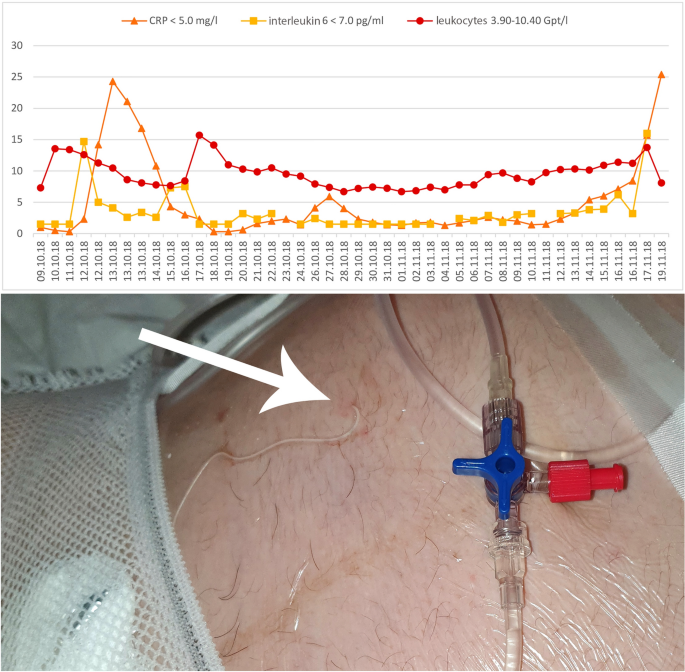
Monitoring of maternal C-reactive protein (CRP), interleukin-6 and leucocytes after preterm premature rupture of membranes (PPROM). The arrow points to the skin's puncture with the implanted prenatal 'anker' catheter
Appropriate counseling was provided including risks and benefits of preterm birth for the fetus and mother, conservative management versus continuous amniotic infusion via a perinatal catheter system with Amnion Flush Solution (2400 ml daily) combined with intrauterine administration of antibiotics (clindamycin 600 mg, Fresenius Kabi, Bad Homburg, Germany) [2, 6, 7]. The implantation of a perinatal 'anker' catheter was performed at the 23/2 WG (Fig. 1, day 0) with the permission of the Martin Luther University Halle-Wittenberg's ethics committee, and written informed patient consent [2, 7].
At 23/6 WG, a cervical bacterial swab culture showed colonization by multi-resistant 3-MRGN E. coli sensitive to meropenem. This antibiotic is listed as Category B in pregnancy [10]. We could not find any information about the intraamniotic use of meropenem and its local effect on the fetus and amniotic membrane. Only a little information was available in relation to meropenem's effect on neonates [11, 12].However, a low trans-placental passage of meropenem is known [10]. Appropriate counseling was provided with neonatology and infectiology teams including the risks and benefits for fetus and mother. The possibility of intraamniotic meropenem application was discussed with the family before getting written informed consent.
Meropenem (Eberth Pharma, Germany) was administered for ten days at 500 mg/day using the perinatal catheter system into the amniotic cavity and 1000 mg three times/day intravenously (Table 1).
Sensitive to the antibiogram, ciprofloxacin (Ciprobay®, Bayer Vital, Germany) was administered for eight days at 400 mg/day through the into the amniotic cavity and 500 mg twice a day per os. The therapy with meropenem was restarted because of a positive multi-resistance 3-MRGN E. coli culture.
The patient gave birth to a male infant via cesarean section at 28/3rd WG (birth weight 1480 g, Apgar score of 7/7/7 at 1/5/10 min, umbilical venous pH 7.49, BE-3) because of increased inflammation parameter Interleukin-6 and CRP without maternal fever. The neonate was transferred to neonatal intensive care unit for further diagnostic and therapeutic management. The newborn required a less invasive surfactant application (Alfeofact®, Lyomark Pharma) followed by non-invasive ventilator support for five days. At admission, Interleukin-6 and CRP values were within normal range. The blood culture as well as throat and umbilical swabs were sterile. The meropenem treatment of the newborn was discontinued at the third day because no clinical signs of neonatal infection were displayed during the period of hospitalization. The neonate was discharged weighing of 3606 g in good condition. The histological examination of amniotic membranes and placenta (weight 405 g, size 14 cm × 13 cm × 3 cm), showed no signs of chorioamnionitis. Follow-up examination at 24 months confirmed normal development without any signs of neurological disturbance.
Discussion and literature review
The very early delivery (prior to 28 weeks of gestation) of premature babies is a worldwide health problem also in developed countries as it is associated with a very high neonatal mortality rate as well as an increased risk of long- and short-term severe neonatal morbidity, physical and developmental disabilities, including chronic respiratory disease (RDS), neurodevelopmental or behavioral effects (impairment of visual/hearing/executive functioning, global developmental delay and psychiatric/behavioral sequela) and cardiovascular diseases [2, 13, 14].
Mid-trimester preterm premature rupture of membranes (PPROM) affects women during the second trimester of their pregnancy. About 0.4–0.7% of all pregnancies are affected by this complication [2]. The causes of the mid-trimester PPROM are multifactorial including local infiltration by bacteria with reaction of pro-inflammatory cytokines, pathologic anatomical remodeling of the amniotic membrane (contribution of MMPs), invasive procedures and fetoscopic surgery, genetic and iatrogenic factors, smoking, vaginal bleeding etc. [2].
Altered membrane morphology including marked swelling and disruption of the collagen network which is seen with PPROM can be triggered by bacterial products or/and pro-inflammatory cytokines [2]. The "classic PPROM" with oligo/ anhydramnion is associated with short latency period and worse neonatal outcome compared to similar gestational aged neonates delivered without antecedent PPROM [2].
Even though the survival rate of premature infants born before 28 weeks of gestation has improved significantly over the last several decades, extreme preterm delivery is still often associated with subsequent neonatal death prior to 1 month of age [15, 16]. Crane et al. [13] published the outcome of infants in Canada delivered at 23 weeks' gestation. The perinatal mortality was 89.9% and of live born neonates admitted to NICU neonatal death occurred in 63.8%. Among survivors at discharge, the rate of severe brain injury was 44.0%, of retinopathy of prematurity 58.3%, and of any serious neonatal morbidity 100% [13]. More than 40% of surviving neonates following PPROM prior to 25 weeks of gestation develop bronchopulmonary dysplasia (BPD) [2].
Prolonged anhydramnion after PPROM is associated with a four-fold increased risk of composite adverse outcomes, including death, BPD, severe neurological disorders, severe retinopathy, when compared to an age-adjusted control group [15, 16].The reaccumulation of the amniotic fluid could improve the neonatal outcome [17].
Spreading of bacterial products and/or pro-inflammatory cytokines trigger alteration of membrane morphology including marked swelling and disruption of the collagen network which is seen with PPROM [2].
Along with expectant management and antenatal corticosteroids, broad-spectrum antibiotics are routinely used with relative limited success in mid-trimester PPROM to prevent bacteremia, chorioamnionitis and FIRS [2, 3]. The trans-placental transfer of antibiotics is depend to used medicine. Some antibiotics with large molecules pass through the placental barrier worse compared to penicillin-group [18,19,20,21]. The amniotic membranes and the umbilical cord do not have an effective capillary net and the antibiotics from maternal circulation do not directly reach the bacteria-colonized surfaces in sufficient concentrations [2].
In our case, the pregnancy was complicated by multi-drug resistant E. coli colonization after the classic PPROM with anhydramnion. E. coli is a bacteria, often isolated to a hospital setting [22]. We report the first combination of continuous amnioinfusion with Amnion Flush Solution 100 ml/h and intraamniotic meropenem application for the treatment of PPROM with multi-resistant E. coli colonization and prophylaxis of FIRS. The i.v. administration of meropenem to the patient was discussed. The majority of investigators as well the patient took a decision to perform a combined i.v. and intraamniotic meropenem applications. In our opinion, the systemic administration of the antibiotic as well as the change of antibiotic related to the antibiogram is a general problem of the sufficient PPROM treatment and should be discussed with specialists in any specific case.
A literature search in Pubmed for "amnioinfusion" in different search contexts revealed a number of 82 hits (including some doubles) resulting in 28 relevant publications, consisting of reviews, randomized clinical trials (RCTs), non-randomized/observational clinical trials and case studies, outlining the state of the art of serial or continuous transabdominal amnioinfusion in most cases using standard infusion solutions who are used off-label in this regard.
Roberts et al., demonstrated that repetitive transabdominal amnioinfusions with Ringer's lactate solution did not improve the perinatal outcome of PPROM patients. In their study, 14 neonates died in the amnioinfusion group versus nine in the control group [23]. Van Kempen et al. [24] (PPROMEXIL-III trial) also did not find any reduction in perinatal mortality after repetitive amnioinfusion with Ringers lactate solution in women with mid-trimester PPROM with oligohydramnion.
A group from Japan demonstrated 2020 that continuous amnioinfusion with Ringer's lactate solution (40 ml/h) significantly increased the PPROM delivery interval by 2 weeks without improving the neonatal outcome [8]. This could be partly explained by a very low 'flush-out' effect because of the high frequency of catheter dislocation (60%) and lack of pump infusion in many cases [8, 9].
Fetal skin in second trimester is still very thin and permeable. It is a matter of common knowledge that the fetus swallows and inspirates/expirates amniotic fluid.
Gilbert and Brace published that the fetus swallows 200–250 ml/kg/day amniotic fluid [25].Continuous amnioinfusion with Normal saline solution significantly increased plasma Na+ and Cl− concentrations in fetal sheep [26].
The amniotic fluid is a very complex hypoosmotic solution with an alkaline pH, low concentration of the elements Cl−, K+ and Na+, presence of trace elements, growth factors and surfactants etc. [27]. In our opinion, the change of physiological fetal surroundings for a long period using amnioinfusion with simple electrolyte solutions could destroy in all probabilities the fetal programming. Osmotic demyelization disorders, central pontine and extra-pontine myelinolysis are well known [28]. Chhabra et al. [29] described the extra-pontine myelinolysis induced by hypernatremia. The combination of very immature brain-blood barrier of the fetus with the permeable skin and swallowing of relative large amount of electrolyte solution deteriorates the fluctuations of the NaCl concentration of the fetal brain. This could explain the high neonatal mortality rate after serial amnioinfusion in the Roberts et al., study and very moderate success of continuous amnioinfusion using Ringer's lactate solution (inappropriate to human amniotic fluid) described in the retrospective Japanese studies [8, 9, 23, 30].
Tchirikov et al. [31] described the use of pump-regulated (100 ml/h) continuous intraamniotic lavage using simple electrolyte solutions through the catheter without fixation since 2008. The dislocation rate of the catheter was very high, until the catheter was improved by an anchor fixation [7]. In our case we decided to replace the anchor catheter after two weeks avoiding the possible local skin inflammation. Last 2 years we replace the catheter normally after one month. In cases of the local skin reaction it must be replaced, too. The patients in whom Jonosteril® (Fresenius Kabi GmbH, Germany), Sterofundin® and isotonic NaCl (B. Braun AG, Melsungen, Germany) or lactated Ringer's solution (Baxter, Germany) were used, reported significantly increased diuresis, probably trans-planetary, triggered by a fetal response to increased NaCl, fluctuation of osmolality and missing microelements also referred to as 'Salzgurken' effect [2]. The electrolyte solutions for the continuous amnioinfusion were replaced 2012 by artificial amniotic fluid (now Amnion Flush Solution, CE0483, Serumwerk AG, Bernburg, Germany) without any adverse events [2, 6, 7]. In 2020, the German Federal Ministry of Education and Research funded a prospective multicentre randomized study investigating the effect of continuous amnioinfusion (22/0-26/0 WG) with Amnion Flush Solution compared with the standard PPROM therapy (ClinicalTrials.gov: NTC04696003).
References
-
Weiner E, Barrett J, Zaltz A et al (2019) Amniotic fluid volume at presentation with early preterm prelabor rupture of membranes and association with severe neonatal respiratory morbidity. Ultrasound Obstet Gynecol 54:767–773. https://doi.org/10.1002/uog.20257
-
Tchirikov M, Schlabritz-Loutsevitch N, Maher J et al (2018) Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. J Perinat Med 46:465–488. https://doi.org/10.1515/jpm-2017-0027
-
Berger R, Abele H, Bahlmann F et al (2019) Prävention und Therapie der Frühgeburt. Leitlinie der DGGG, OEGGG und SGGG (S2k-Niveau, AWMF-Registernummer 015/025, Februar 2019)—Teil 2 mit Empfehlungen zur tertiären Prävention der Frühgeburt und zum Management des frühen vorzeitigen Blasensprungs (Prevention and Therapy of Preterm Birth. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry Number 015/025, February 2019)—Part 2 with recommendations on the tertiary prevention of preterm birth and the management of preterm premature rupture of membranes). Z Geburtshilfe Neonatol 223:373–394. https://doi.org/10.1055/a-1008-8730
-
Chatzakis C, Papatheodorou S, Sarafidis K et al (2020) Effect on perinatal outcome of prophylactic antibiotics in preterm prelabor rupture of membranes: network meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 55:20–31. https://doi.org/10.1002/uog.21884
-
Gomez R, Romero R, Nien JK et al (2007) Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med 20:167–173. https://doi.org/10.1080/14767050601135485
-
Tchirikov M, Zhumadilov Z, Winarno AS et al (2015) Treatment of preterm premature rupture of membranes with oligo-/anhydramnion colonized by multiresistant bacteria with continuous amnioinfusion and antibiotic administrations through a subcutaneously implanted intrauterine port system: a case report. Fetal Diagn Ther. https://doi.org/10.1159/000438483
-
Tchirikov M, Bapayeva G, Zhumadilov ZS et al (2013) Treatment of PPROM with anhydramnion in humans: first experience with different amniotic fluid substitutes for continuous amnioinfusion through a subcutaneously implanted port system. J Perinat Med 41:657–663. https://doi.org/10.1515/jpm-2012-0296
-
Ono T, Tsumura K, Kawasaki I et al (2020) Continuous amnioinfusion for treatment of mid-trimester preterm premature rupture of membranes with oligoamnios. J Obstet Gynaecol Res 46:79–86. https://doi.org/10.1111/jog.14151
-
Esaki M, Maseki Y, Tezuka A et al (2020) Continuous amnioinfusion in women with PPROM at periviable gestational ages. J Matern Fetal Neonatal Med 33:1151–1156. https://doi.org/10.1080/14767058.2018.1517307
-
Hnat M, Bawdon RE (2005) Transfer of meropenem in the ex vivo human placenta perfusion model. Infect Dis Obstet Gynecol 13:223–227. https://doi.org/10.1080/10647440500147992
-
Esposito S, Pinzani R, Raffaeli G et al (2016) A young infant with transient severe hypertriglyceridemia temporarily associated with meropenem administration: a case report and review of the literature. Med (Baltim) 95:e4872. https://doi.org/10.1097/MD.0000000000004872
-
Velaphi S, Wadula J, Nakwa F (2009) Mortality rate in neonates infected with extended-spectrum beta lactamase-producing Klebsiella species and selective empirical use of meropenem. Ann Trop Paediatr 29:101–110. https://doi.org/10.1179/146532809X440716
-
Crane JMG, Magee LA, Lee T et al (2015) Maternal and perinatal outcomes of pregnancies delivered at 23 weeks' gestation. J Obstet Gynaecol Can 37:214–224
-
Pendse A, Panchal H, Athalye-Jape G et al (2020) Neonatal outcomes following previable prelabour rupture of membranes before 23 weeks of gestation- a retrospective cohort study. J Neonatal Perinatal Med. https://doi.org/10.3233/NPM-190366
-
Manuck TA, Varner MW (2014) Neonatal and early childhood outcomes following early vs later preterm premature rupture of membranes. Am J Obstet Gynecol 211:308.e1–6. https://doi.org/10.1016/j.ajog.2014.05.030
-
Soylu H, Jefferies A, Diambomba Y et al (2010) Rupture of membranes before the age of viability and birth after the age of viability: comparison of outcomes in a matched cohort study. J Perinatol 30:645–649. https://doi.org/10.1038/jp.2010.11
-
Schierlitz L, Barker GK, Walker SP et al (2001) Successful pregnancy outcome after preterm premature rupture of membranes at < 20 weeks. A report of three cases. J Reprod Med 46:263–266
-
Onwuchuruba CN, Towers CV, Howard BC et al (2014) Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol 210:352.e1–4. https://doi.org/10.1016/j.ajog.2014.01.019
-
Park HS, Ahn B-J, Jun JK (2012) Placental transfer of clarithromycin in human pregnancies with preterm premature rupture of membranes. J Perinat Med 40:641–646. https://doi.org/10.1515/jpm-2012-0038
-
Polachek H, Holcberg G, Sapir G et al (2005) Transfer of ciprofloxacin, ofloxacin and levofloxacin across the perfused human placenta in vitro. Eur J Obstet Gynecol Reprod Biol 122:61–65. https://doi.org/10.1016/j.ejogrb.2004.11.031
-
Viel-Theriault I, Fell DB, Grynspan D et al (2019) The transplacental passage of commonly used intrapartum antibiotics and its impact on the newborn management: a narrative review. Early Hum Dev 135:6–10. https://doi.org/10.1016/j.earlhumdev.2019.05.020
-
Ghodhbane H, Elaidi S, Sabatier J-M et al (2015) Bacteriocins active against multi-resistant gram negative bacteria implicated in nosocomial infections. Infect Disord Drug Targets 15:2–12. https://doi.org/10.2174/1871526514666140522113337
-
Roberts D, Vause S, Martin W et al (2014) Amnioinfusion in preterm premature rupture of membranes (AMIPROM): a randomised controlled trial of amnioinfusion versus expectant management in very early preterm premature rupture of membranes—a pilot study. Health Technol Assess 18:1–135. https://doi.org/10.3310/hta18210
-
van Kempen LEM, van Teeffelen AS, de Ruigh AA et al (2019) Amnioinfusion compared with no intervention in women with second-trimester rupture of membranes: a randomized controlled trial. Obstet Gynecol 133:129–136. https://doi.org/10.1097/AOG.0000000000003003
-
Gilbert WM, Brace RA (1993) Amniotic fluid volume and normal flows to and from the amniotic cavity. Semin Perinatol 17:150–157
-
Shields LE, Moore TR, Brace RA (1995) Fetal electrolyte and acid-base responses to amnioinfusion: lactated Ringer's versus normal saline in the ovine fetus. J Soc Gynecol Investig 2:602–608
-
Fitzsimmons ED, Bajaj T (2021) Embryology, amniotic fluid. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL
-
Brown WD (2000) Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 13:691–697. https://doi.org/10.1097/00019052-200012000-00014
-
Chhabra A, Kaushik R, Kaushik RM et al (2017) Extra-pontine myelinolysis secondary to hypernatremia induced by postpartum water restriction. Neuroradiol J 30:84–87. https://doi.org/10.1177/1971400916678246
-
Roberts D, Vause S, Martin W et al (2014) Amnioinfusion in very early preterm prelabor rupture of membranes (AMIPROM): pregnancy, neonatal and maternal outcomes in a randomized controlled pilot study. Ultrasound Obstet Gynecol 43:490–499. https://doi.org/10.1002/uog.13258
-
Tchirikov M, Steetskamp J, Hohmann M et al (2010) Long-term amnioinfusion through a subcutaneously implanted amniotic fluid replacement port system for treatment of PPROM in humans. Eur J Obstet Gynecol Reprod Biol 152:30–33. https://doi.org/10.1016/j.ejogrb.2010.04.023
Funding
Open Access funding enabled and organized by Projekt DEAL. None to declare.
Author information
Authors and Affiliations
Contributions
MT: protocol/project development, data collection or management, data analysis, manuscript writing/editing. RO: data collection or management, data analysis. gregor seliger: data collection or management, data analysis. KC: data collection or management, data analysis. SM: manuscript editing. RH: data analysis, manuscript editing.
Corresponding authors
Ethics declarations
Conflicts of interest
None to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent from all participants (pregnant women and parents/legally authorized representative of the neonates) was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Tchirikov, M., Ocker, R., Seliger, G. et al. Treatment of mid-trimester preterm premature rupture of membranes (PPROM) with multi-resistant bacteria-colonized anhydramnion with continuous amnioinfusion and meropenem: a case report and literature review. Arch Gynecol Obstet 306, 585–592 (2022). https://doi.org/10.1007/s00404-021-06319-w
-
Received:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1007/s00404-021-06319-w
Keywords
- Amnioinfusion
- Anhydramnion
- 3-MRGN
- E. coli
- Preterm premature rupture of membranes
- PPROM
Source: https://link.springer.com/article/10.1007/s00404-021-06319-w
0 Response to "Continuous Amnioinfusion in Women With Pprom at Periviable Gestational Ages"
Post a Comment